Home » Products » Point Of Care » Diabetes » Atellica DCA Analyzer
Atellica DCA Analyzer
Compact. Portable. Scalable. Customizable.
Building upon one of the industry’s most trusted technologies for diabetes testing, Atellica® DCA Analyzer puts fast, quality HbA1c and ACR results right at your fingertips. Experience the performance and precision you need to improve outcomes. Designed with the speed, standardization, and personalization you want to boost efficiencies and meet growing demand.
Compact and portable
With a small footprint Atellica DCA Analyzer can be placed wherever it is needed when space is at a premium.


Intuitive UI and flexible workflow
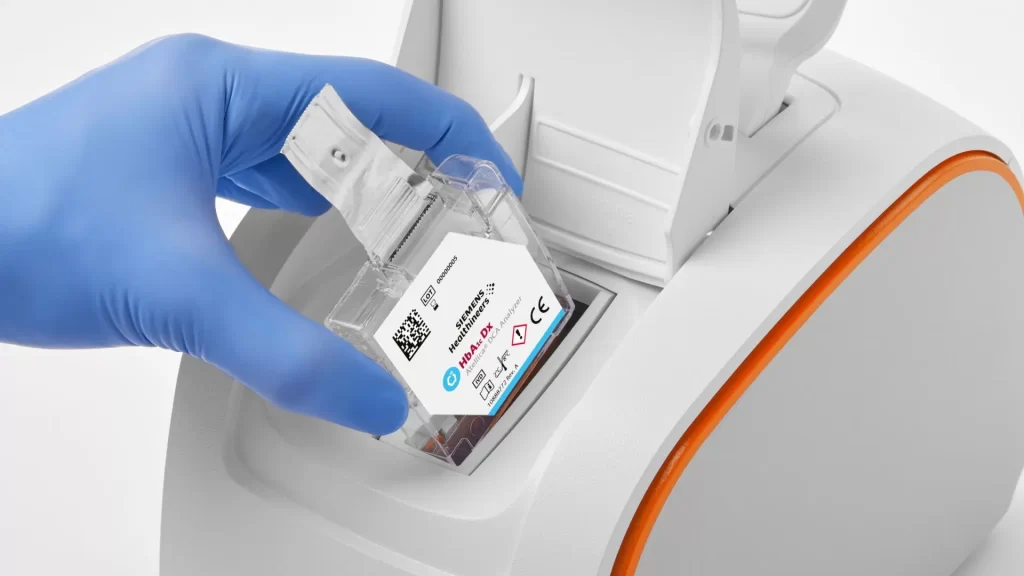
Use patient-sample ID link to help mitigate the risk of misidentifying samples. Bring the handheld display directly to the patient, to scan and link the patient and cartridge information.
Atellica DCA Analyzer was designed to provide confidence with every test. Simple operation and intuitive user interface (UI) decrease the likelihood of operator error, improves user experience, and reduces training complexity.
Trusted performance
HbA1c results available in minutes can help ensure timely patient consultation and treatment paths. ACR results in only 7 minutes can help aid in the early diagnosis of kidney disease.
Building on 30+ years of innovation in HbA1c testing, our industry-leading immunoassay technology delivers reliable performance and excellent precision across the entire assay range, ensuring confidence test after test. Expand your diabetes management program to better serve your clinical staff and patients with Atellica DCA Analyzer.

Improved patient experience
Face-to-face counseling enables education, helps to engage patients, and leads to increased compliance with treatment plans.
Scalable and connected
Connect and operate up to three test modules with a single display to accommodate increasing testing volume.

System Description
Point-of-care immunoassay analyzer
Quantitative Tests
Hemoglobin A1c (whole blood)
4.0–14% (20–130 mmol/mol)
ACR (urine)
Albumin: 5–300 mg/L
Creatinine: 15–500 mg/dL (1.3–44.2 mmol/L)
Albumin-to-creatinine ratio (ACR): 1–2000 mg/g (0.1–230.8 mg/mmol)
Test Format
Self-contained immunoassay cartridges
Test Measurement
Automatic optical transmission
Test Results Units
Measured Parameters
HbA1c: %HbA1c (NGSP/DCCT) and mmol/mol (IFCC)
Albumin: mg/dL
Creatinine: mg/dL or mmol/L
Calculated Parameters
ACR: mg/g or mg/mmol
Test Method
HbA1c: monoclonal antibody agglutination reaction
Albumin: monoclonal antibody agglutination reaction
Creatinine: Benedict Behre chemical reaction
Time to Results
HbA1c: 5 minutes
ACR: 7 minutes
Sample Volume
HbA1c: 1 µL whole blood; capillary or venous (K2 EDTA, Li heparin, Na citrate)
ACR: 40 µL urine
Sample Prep
No pretreatment or pipetting required
Sample ID, Operator ID Entry
Optional, via touchscreen or built-in bar-code scanner
HbA1c Blood Sample Hold Time
4 minutes
Why Choose Us
We believe that success is achieved with professionalism, integrity and perfect teamwork, built on the high moral values of our employees.
Our policy is based on impeccable planning, control and execution, through which we achieve maximum satisfaction of our customers.
Professional
customer service
A wide range of laboratory analyzers
Trained and experienced specialists
Certificate
ISO 13485:2012



