Home » Products » Point Of Care » Cardiac » Atellica® VTLi Patient-side Immunoassay Analyzer
Atellica® VTLi Patient-side Immunoassay Analyzer
A vital leap forward in cardiac testing.
When a patient enters the emergency department presenting with the symptoms of a myocardial infarction (MI), every moment is critical. Achieving favorable clinical and operational outcomes requires fast and accurate chest pain assessment at the point of care, as well as coordination with the laboratory for quality assurance. Having high-sensitivity troponin testing at the point of care—producing accurate results in just 8 minutes—could transform care delivery for millions of people each year, improving ED throughput, reducing stress and strain on acute care clinicians, and aiding in optimizing the use of time and resources of busy laboratories.
Features & Benefits
Improved ED throughput
The Atellica VTLi system streamlines chest pain assessment, aiding in earlier disposition decisions for patients not having an MI and fast interventions for those who are.


Easily integrated into assessment processes
The Atellica VTLi system will aid in transforming your chest pain assessment process to benefit patients, clinicians, and operational workflow.
Streamlined patient testing workflow
Operator inoculates cartridge with capillary sample and with venous sample
- Sample type flexibility: The system can produce results on lithium-heparin whole blood, lithium-heparin plasma, and capillary samples.
- Sample volume: minimum of 30 µL of blood required to perform the test.
- Test results are sent securely through our POC Ecosystem™ Solution via WI-FI or Ethernet for storage in the LIS/HIS and EMR.

Deliver real-time results and reporting
The cartridge is disposable (single-use), with no warm-up time from refrigerated storage. Each disposable cartridge is identified by a unique Radio Frequency Identification (RFID) chip, that contains information on the type of test, calibration data, and lot-specific information.
- Instructs the instrument to automatically starts the correct assay protocol
- Integrated assay quality control in every cartridge
Technical Specifications
Sample Type
Capillary (fingerstick) whole blood
Lithium-heparinized venous whole blood
Lithium-heparinized venous plasma
Sample Size
30–100 μL
Analysis
Time to Results: ~8 minutes
Quality Control
Sero PATHONORM Cardiac Acute Liq
Reagent Cartridge
Storage: 2–8°C
Size of box (W x D x H): 152 x 95 x 167 mm
Cartridges per box: 24
System Dimensions
Length: 25 cm
Height: 5.2 cm
Width: 8.5 cm
Weight: 780 g
Color LCD display: 7.3 inches
Integrated Barcode Scanner
Patient ID, Operator ID, and bottled QC; 1D (linear) and 2D barcode symbologies
Operating System
Microsoft WEC2013
Data Security
Patient data anonymization
TLS 1.2 encrypted end-to-end communication, using client and server certificates
Configurable level of user authentication
No storage of passwords, no hard-coded passwords
No user access to operating system
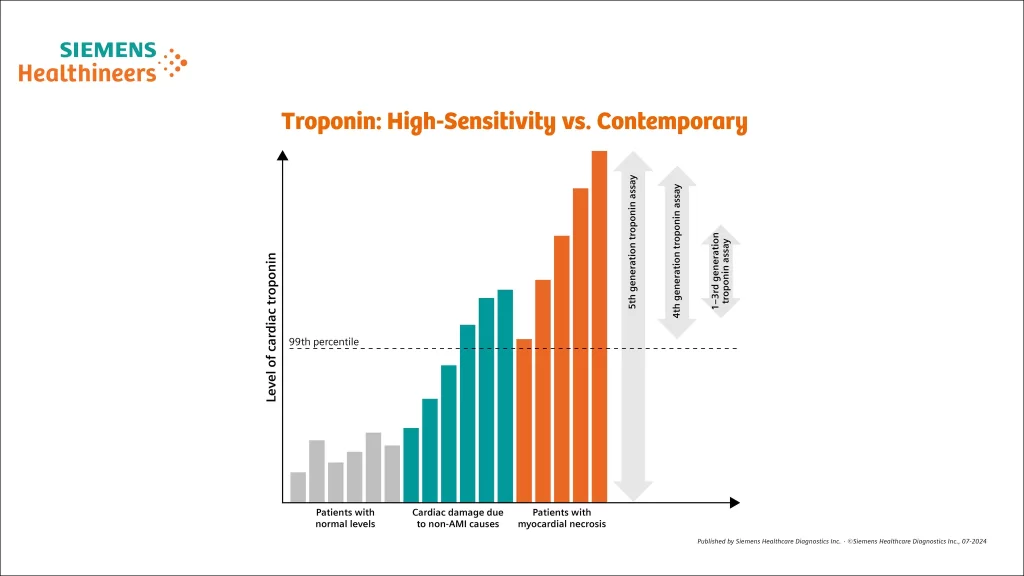
Troponin: High-Sensitivity vs. Contemporary
- Contemporary assays must detect cTn in 20% to <50% of healthy individuals.
- High-sensitivity assays must detect cTn in at least 50% of healthy individuals and must have <10% CV at the 99th percentile of normal.
- Units of measure:
- Contemporary: ng/mL
- High-sensitivity: ng/L
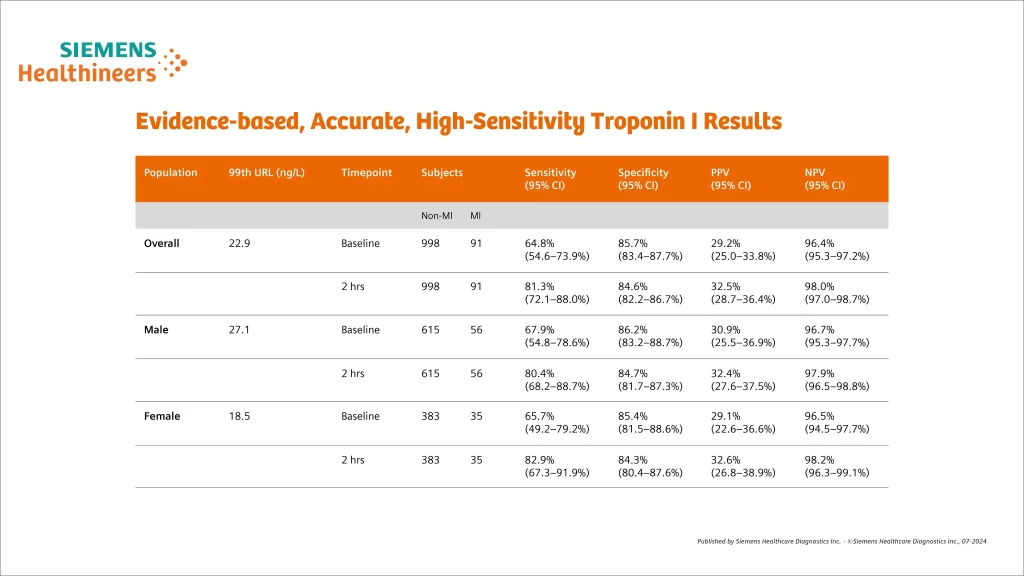
Evidence-based, accurate performance
- Compared with conventional troponin assays:
- Higher NPV for AMI
- Reduced “troponin-blind” interval, leading to earlier detection of AMI
- Result in ~4% absolute and ~20% relative increases in the detection of type 1 MI and corresponding decrease in the diagnosis of unstable angina
- Associated with 2-fold increase in the detection of type 2 M
- Levels of hs-cTn are quantitative markers of cardiomyocyte damage:
- Elevations greater than 5 times the URL have high (90%) PPV for acute type 1 MI.
- Elevations up to 3 times the URL have limited (50–60%) PPV for AMI.
- It is common to detect circulating levels of cardiac troponin in healthy people.
- Rising and/or falling cardiac troponin levels differentiate acute from chronic cardiomyocyte damage.
Why Choose Us
We believe that success is achieved with professionalism, integrity and perfect teamwork, built on the high moral values of our employees.
Our policy is based on impeccable planning, control and execution, through which we achieve maximum satisfaction of our customers.
Professional
customer service
A wide range of laboratory analyzers
Trained and experienced specialists
Certificate
ISO 13485:2012