Home » Products » Integrated Clinical Chemistry and Immunoassay Analyzers » Atellica CI Analyzer
Atellica CI Analyzer
The Atellica CI Analyzer addresses big challenges—all in a compact 1.9m2 footprint..
The weight of the responsibility you carry in the lab, and the enormity of the challenges you face to deliver quality testing– quickly, consistently, and accurately – day after day, is constant. Labs of all sizes are being asked to do more with less. This calls for more than a one-size fits all approach. The Atellica® CI Analyzer addresses these issues head-on—achieve predictable patient care, deliver greater agility across your network and meet security and sustainability goals – all in a compact 1.9m2 footprint.
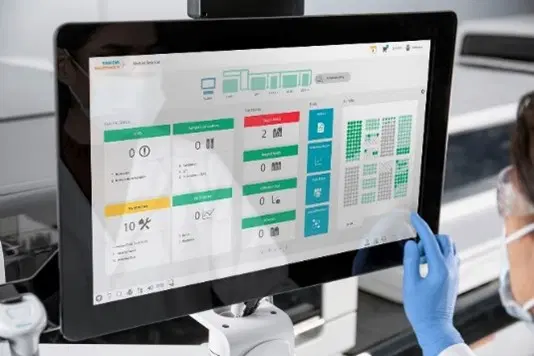
Sophisticated User Interface
Integrated with common laboratory functions to help minimize training and ensure optimal workflow
- Easy for every skill level. With workforce shortages primary operators may have varied backgrounds. The Atellica® CI Analyzer is designed so anyone can feel comfortable in a minimal amount of time.
- Customizable for navigation and alerting for your lab. Setup your user interface navigation and set notifications like you setup a smartphone so it fits your laboratory your way.
- Provides Traceability and Inspection Readiness. Your workload is more than just patient results and throughput. The user interface anticipates your needs for accreditation requirements.
Chemistry: Up to 1000 test per hour (600 PMs, 400 ISEs) with 70 reagent positions
- Microvolume technology maximizes the test yield from
a single aspiration. - IMT (Integrated Multisensor Technology) sensor for
electrolyte analysis with automatic QC and no daily maintenance. - Total 70 Chemistry reagent positions accommodating
single & dual reagent kits up to 2100 tests
Immunoassay: Up to 120 tests per hour with 20 primary and 20 ancillary positions
- Environmental controls and consistent temperature for precise immunoassay reactions
- Separate wash ring provides a tailored wash routine for the assay and helps deliver low-end sensitivity and specificity
- Total 40 immunoassay reagent positions (20 primary and 20 ancillary reagent positions)

Independent Integration
Sample Handling
The rack handler has both input and output queues and can accommodate 60 samples in 10 six (6) position racks with STAT testing capabilities.


Standardized to Atellica Solution
Atellica® CI Analyzer uses the same technology, workflow, reagents, consumables, and user interface, all in 1.9 m2.
Operational Efficiency
Independent Integration and Random-Access Sampling maximizing throughput up to 1120 tests per hour.
- Maintenance: <5 minutes hands-on daily maintenance.
- STAT Testing: STAT sample processing within in 60 seconds.
- Consumables: load/unload reagents and system consumables on the fly, and during sample processing.
- Standardized to Atellica Solution: providing continuity across the network and sustainability efforts across Siemens Healthineers.
Chemistry
- Microvolume technology maximizes the test yield from a single sample aspiration
- IMT sensor for electrolyte analysis with automatic calibration
- Total 70 chemistry reagent positions, accommodating single and dual well reagent packs, with up to 2100 tests per kit
- Up to 6 months reagent onboard stability
- Calibration intervals up to 200 days
Immunoassay
- Environmental controls and consistent temperatures for precise immunoassay reactions
- Temperature controlled incubation, fast throughput for efficient turn around times
- Separate wash ring provides a tailored wash routine for each assay and helps deliver low end sensitivity, and specificity.
- Total 40 immunoassay reagent positions (20 primary and 20 ancillary positions)
- Up to 3 months reagents onboard stability
- Calibration intervals up to 90 days
Maximize your lab’s potential with high-performing assays that deliver what matters most ─ accurate, clinically relevant results on an efficient and scalable instrument:
- Full cardiac panel, including your choice of BNP or NT-proBNP, and high-sensitivity troponin I, the only hs-cTnI assay with faster protocols validated in APACE, High-STEACS and HIGH-US cohorts.
- Anti-Müllerian Hormone (AMH) for the assessment of ovarian reserve, part of a growing reproductive endocrinology menu that also includes sFlt-1*and PlGF* to aid in the prognosis of preeclampsia.
- Comprehensive infectious disease menu with workflow-efficient HIV and hepatitis assays, and the only HBsAg II* assay that offers a “hot zone” and SMART algorithm for greater lab efficiency.
- Proprietary ELF™ Test*, a simple blood test to evaluate the risk of NASH liver fibrosis progression.
With a planned menu of over 200 assays across 20 disease states, the Atellica CI Analyzer helps standardize results and streamline workflows for your entire network. When results, workflows, and inventory are standardized across users and locations, you protect time and budgets to help every lab in your network increase the value of its care.
*Under development. Not available for sale.
Product Specifications
Description
Integrated chemistry and immunoassay analyzer. Chemistry and immunoassay technologies share
no major components to help maximize test throughput while minimizing laboratory footprint.
Test Throughput
Up to 1120 tests per hour (up to 600 photometric, 400 IMT, 120 immunoassay)
User Interface
Integrated software with intelligent monitoring of supplies, consumables, and common laboratory
tasks from the home screen dashboard. User interface includes onboard tools to assist with
laboratory accreditation and simplifies training to optimize the user experience with guided
workflows, lab evaluation suite, and customizable dashboard.
Walkaway Time
2 hours
Sample Handling
Validated Sample Types
Serum, plasma, amniotic fluid, urine, whole blood (assay-specific), CSF, and other
Sample Integrity Control
Liquid-level sensing, clot/clog detection, bubble detection, short-sample detection; hemolysis, icterus, and lipemia
Auto-repeat
Automatic repeat testing from the original and diluted samples
Sample Dilution
Assay-dependent; can be auto-diluted and repeated when results extend linearity
Auto-reflex Testing
Configurable; additional tests based on results of first test or test mix
Sample Carryover Prevention
Chemistry uses precision wash system.
Immunoassay uses disposable sample tips to eliminate carryover.
Sample Volume per Test
2–100 μL of sample (varies by assay)
Reaction Area
Reaction Cuvettes
CH dilution cuvettes (64 reusable cuvettes: four segments with 16 cuvettes each)
CH reaction ring segments (clinical chemistry = 130 reusable cuvettes, 10 segments, 13 cuvettes)
IM incubation ring holds 56 cuvettes.
Reaction Temperature
CH: 37°C ±0.3°C, IM: 37°C ±0.4°C
Chemistry Reaction Detection
Reaction area: photometer, LED light source with 11 fixed wavelengths (340, 410, 451, 478, 505,
545, 571, 596, 658, 694, 805 nm). Linearity: 0–3.0 AU. Resolution: 0.0001 AU.
Chemistry Assay Calculations
Endpoint (EPA), rate reaction (RRA), 2-point rate (2PA), sample blank correction
Immunoassay Reaction Detection
Photomultiplier tube
Immunoassay Reaction Formats
Sandwich, competitive, and antibody-capture/antigen-bridge formats
Assay Time
Chemistry and immunoassay: 1–54 minutes, assay-dependent
Assay Technology
CH: Integrated Multisensor Technology (IMT, electrolytes), photometric, and turbidimetric
IM: chemiluminescence testing methodology using advanced acridinium ester technology
Reagent Handling
Reagent Compartments
CH: one tray (70 positions), refrigerated, temperature-controlled compartment 4–12°C
IM: 20 primary, 20 ancillary reagent positions with refrigeration and humidity control, continuous
and automatic mixing to maintain particle suspension, temperature-controlled compartments 4–10°C
Reagent Packs
CH: 50 mL dual-well reagent containers (2 x 25 mL each); 95–2100 tests per pack
IM: ReadyPack® cartridge: 50–200 tests per pack
Reagent Integrity Control
Reagent pack barcode identification: automatic pack/well tracking, notification of inventory, calibration
and control validity, onboard stability, low and expired reagents, detection of reagent bubbles
Onboard Stability
CH: up to 6 months (assay-dependent)
IM: up to 3 months (assay-dependent)
Dispensing System
CH: one probe with liquid-level sensing and crash detection
IM: one probe with liquid-level sensing and crash detection
Barcode-labeled Packs
Yes
Average Reagent Volume
10–100 μL per test, assay-dependent
Open Channels
CH only: available. Configurable to assay specifications with ability to copy Atellica CH assays
and configure per laboratory needs.
Integrated Multisensor Technology (IMT) for Na+, K+, Cl− (CH only)
Assay Time
18 seconds
Sample Volume
25 μL produces three results
Sample Dilution
Automatic 1:10; automatic monitoring for bias with every patient result
Calibration
Automatic calibration
Priming
Automatic priming cycle
A-LYTE™ Integrated Multisensor
Technology Cartridge Use Life
Up to 5000 samples or 14 days
Calibration/QC
Auto-calibration
Automatic calibration orders generated by test definition for CH and IM assays.
Calibration Review
Graphical display of calibration curves for a minimum
of 20 different reagent lots and 20 reagent packs for
each assay
with autovalidation.
Auto-QC
Automatic QC orders generated by test definition for CH
and IM assays. Quality control testing can be
automatically
ordered by day, time, panel, test count, control material,
and with calibration orders.
Quality Control Review
Advanced QC package with graphical display of QC in real time,
including patient moving averages, Levey-Jennings plots,
Westgard rules, RiliBÄK rules; up to 125,000 control results can
be stored; autovalidation, archivable to removable media.
QC/Calibration Material
QC and calibration materials are tracked in the software by test
definition and sequence number.
Includes onboard stability and
interval expiration.
Laboratory Evaluations
Patent-pending assay evaluation suite. Provides onboard
support for precision testing, automatic and manual measuring
interval verification studies, QC parallel and reagent lot-to-lot
testing.
Maintenance
Daily
Hands-on: <5 minutes; automated: ≤30–45 minutes
Weekly
Hands-on: <4 minutes; automated: up to 75 minutes
Monthly
Hands-on: <5 minutes
As Needed
Refer to online help for additional periodic maintenance.
Logs
Operator, Maintenance, LIS, and Audit Trail logs monitor
activities via the software.
Monthly approvals, stored on the
system, printable, exportable, and formatted for inspections.
General Specifications
Power Requirements
Voltage: 200–240 VAC, current: 24 A, frequency: 50/60 Hz
5.0 m power cord with
IEC 60309 (6H) 30A/250V 2P+E plug
(OUS) or NEMA L6-30 plug (U.S.)
IEC 60309 (6H) 30A/250V 2P+E receptacle required (must be supplied by the facility).
Power Consumption
2.2 kW
Water Input Requirements
Incoming pressure 5–30 psi at 10–30°C
Water Quality Requirements
Special reagent water (SRW) required:
• Resistivity: ≥10 MΩ/cm
• Bacteria: ≤50 CFU/mL
• Total organic carbon: ≤500 ppb
A 0.22 micron filter is required at the output stage of the
laboratory.
An additional 0.22 micron filter is required before
the input to the water supply.
Maximum Water Consumption
Up to 25 liters of water per hour at maximum instrument capacity.
Drain Requirements
76.2 mm (3 in.) drain is recommended to handle minimum of 100 L/hour (1.7 L/min).
Dimensions with Rack Handler
(H) 1600 mm, (W) 2034 mm, (D) 934 mm = <1.9 m2
Weight
760 kg (1675.5 lb)
Compliance
Complies with international environmental, health, and safety standards, including CE and RoHS.
Noise Emission
Complies with NC-43 noise control specification. Average sound pressure of <65 dBA 1 m from analyzer.
Processing Heat Output
7500 BTU/hr
Ambient Temperature
18–30°C (64–86°F)
Ambient Humidity
20–80% noncondensing
Altitude
Up to 2000 meters
Floor Load-bearing Requirement
400 kg/m2, seismic anchoring available
Overvoltage Classification
Category II
Pollution Classification
Degree 2
Removable Media
USB
Why Choose Us
We believe that success is achieved with professionalism, integrity and perfect teamwork, built on the high moral values of our employees.
Our policy is based on impeccable planning, control and execution, through which we achieve maximum satisfaction of our customers.
Professional
customer service
A wide range of laboratory analyzers
Trained and experienced specialists
Certificate
ISO 13485:2012



